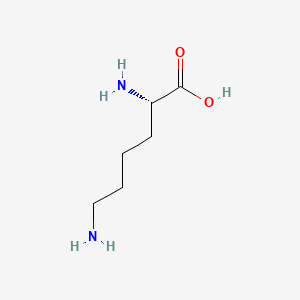
Molecules | Free Full-Text | Structural and Mechanistic Basis for the Inactivation of Human Ornithine Aminotransferase by (3S,4S)-3-Amino-4-fluorocyclopentenecarboxylic Acid
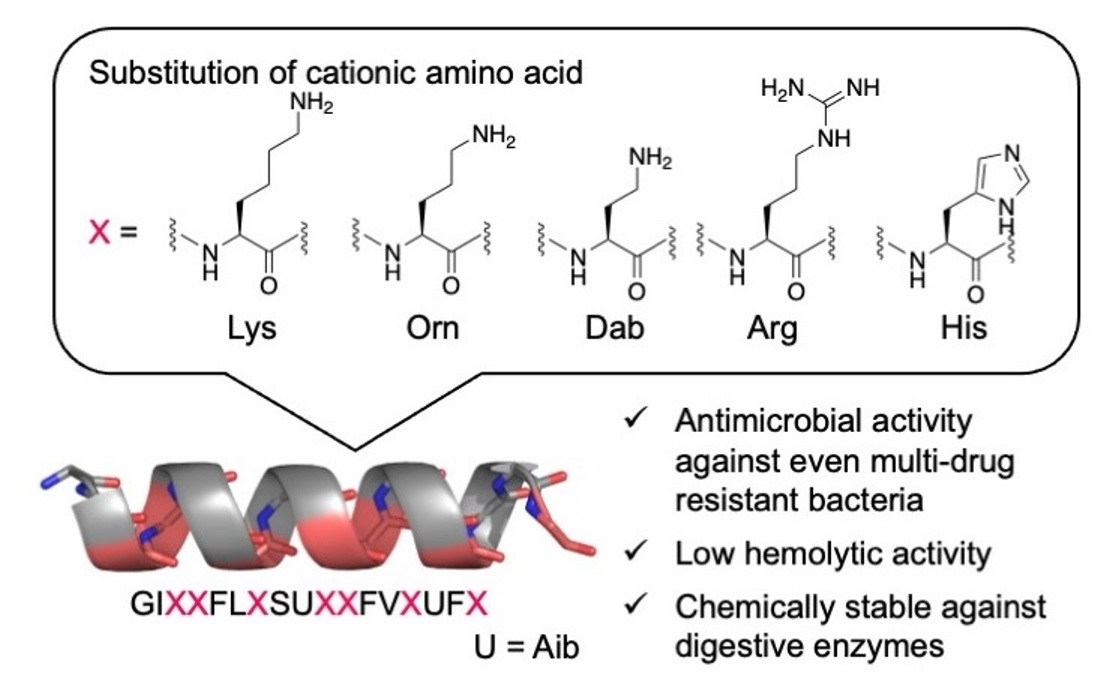
Antibiotics | Free Full-Text | Structure–Activity Relationship Studies of Substitutions of Cationic Amino Acid Residues on Antimicrobial Peptides

KR20120093310A - 2-amino-3-methyl-hex-5-enoic acid and its use in the production of peptides such as bacitracins - Google Patents

Biosynthesis of the 22nd Genetically Encoded Amino Acid Pyrrolysine: Structure and Reaction Mechanism of PylC at 1.5 Å Resolution - ScienceDirect
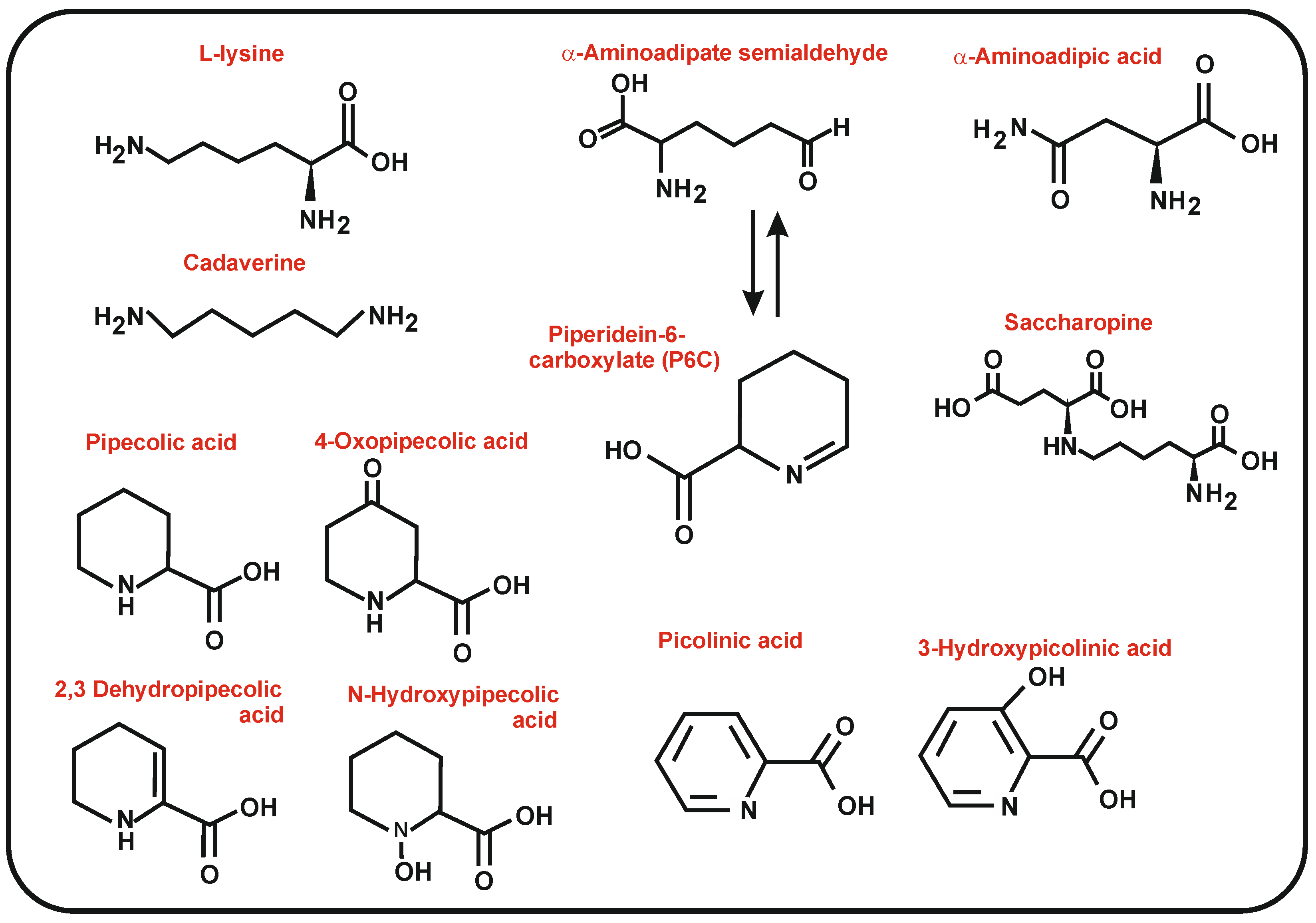
Antibiotics | Free Full-Text | Interconnected Set of Enzymes Provide Lysine Biosynthetic Intermediates and Ornithine Derivatives as Key Precursors for the Biosynthesis of Bioactive Secondary Metabolites

Molecules | Free Full-Text | GC-MS Discrimination of Citrulline from Ornithine and Homocitrulline from Lysine by Chemical Derivatization: Evidence of Formation of N5-Carboxy-ornithine and N6-Carboxy-lysine
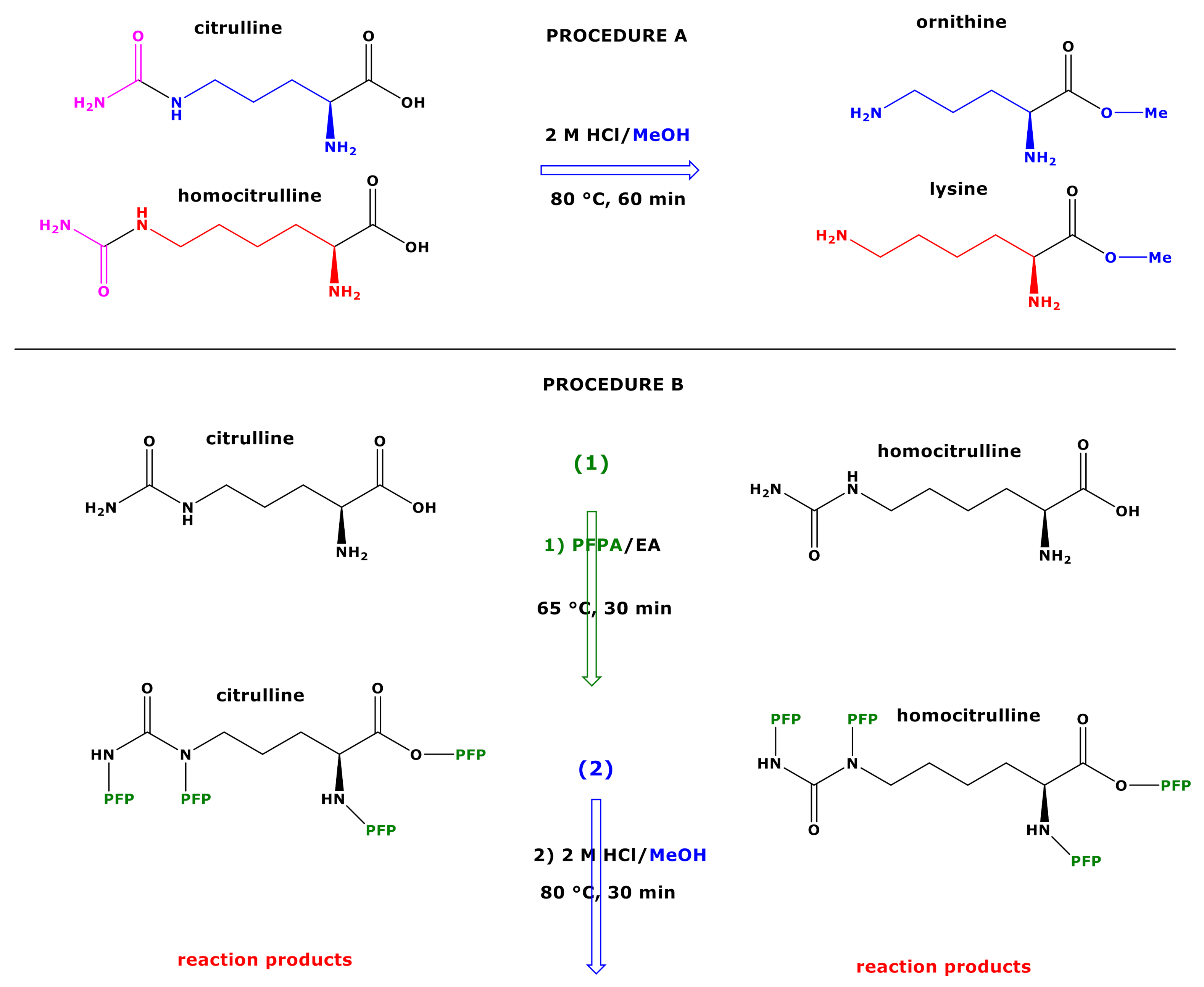
Molecules | Free Full-Text | GC-MS Discrimination of Citrulline from Ornithine and Homocitrulline from Lysine by Chemical Derivatization: Evidence of Formation of N5-Carboxy-ornithine and N6-Carboxy-lysine

Cell-Penetrating Peptides Using Cyclic α,α-Disubstituted α-Amino Acids with Basic Functional Groups | ACS Biomaterials Science & Engineering

KR20120093310A - 2-amino-3-methyl-hex-5-enoic acid and its use in the production of peptides such as bacitracins - Google Patents

Therapeutic Potential of Foldamers: From Chemical Biology Tools To Drug Candidates? | Journal of Medicinal Chemistry